Bioavailability and Bioequivalence (BA/BE)
We run BA/BE studies that generic manufacturers need to show their formulations perform the same way as reference products in humans.
Overview
BA/BE studies are the backbone of generic drug approval. You dose healthy volunteers with test and reference products, collect blood samples at scheduled timepoints, measure drug concentrations in plasma, and calculate whether the two formulations are bioequivalent. We handle the entire process from protocol writing to final study report.
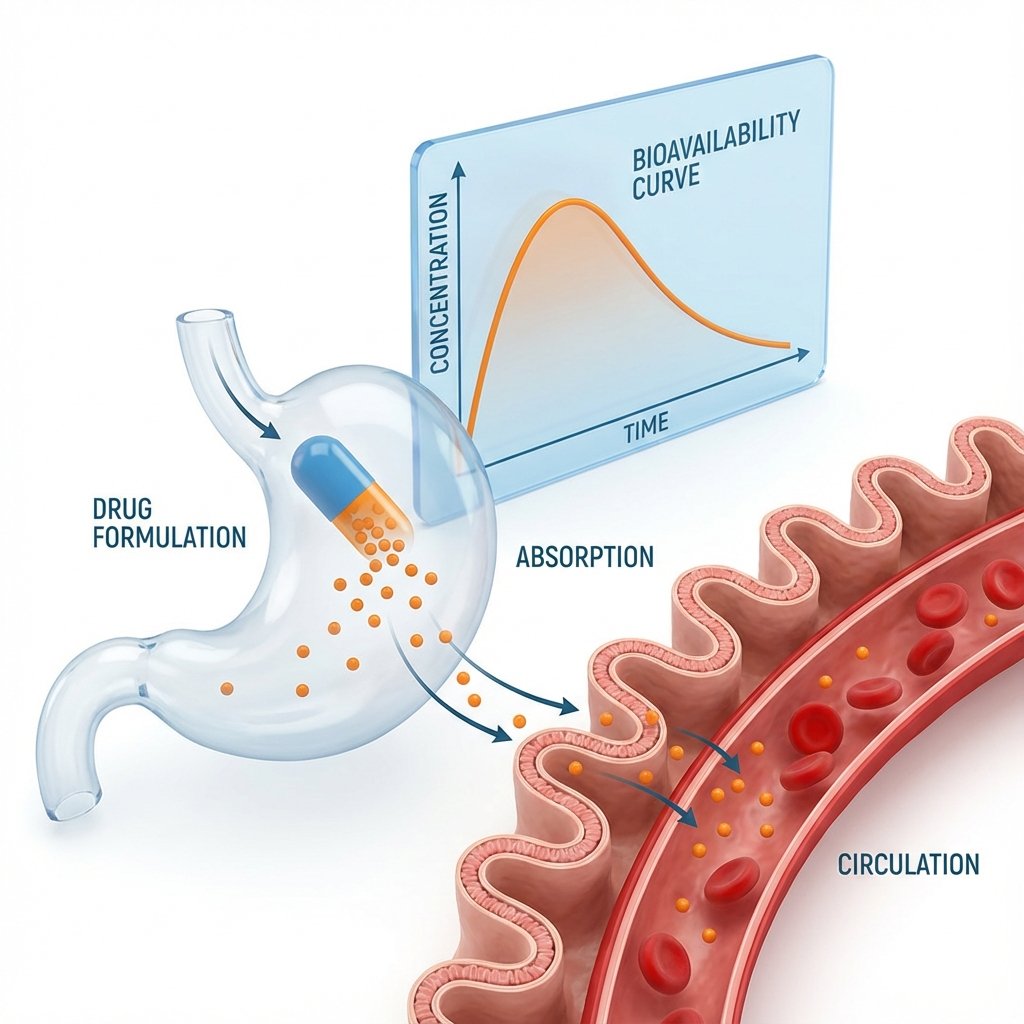
What We Do
Why Work With Us
We are 80 Bedded facility with Four LCMS with 21 CFR 11 compliance
Our clinical facility is equipped for overnight confinement and controlled meal administration
In-house bioanalytical lab means faster turnaround on sample analysis
Ready to Get Started?
Let's discuss how we can support your bioavailability and bioequivalence (ba/be) needs.
